Ethanoic Acid and Sodium Hydroxide
Add drops of this phenolphthalein solution to 100 mL of 05 mol litre sodium hydroxide solution until a deep pink colour appears. Or 10 cm 3 of 10 mol dm-3 sodium ethanoate solution with 10 cm 3 of 20 mol dm-3 ethanoic acid.
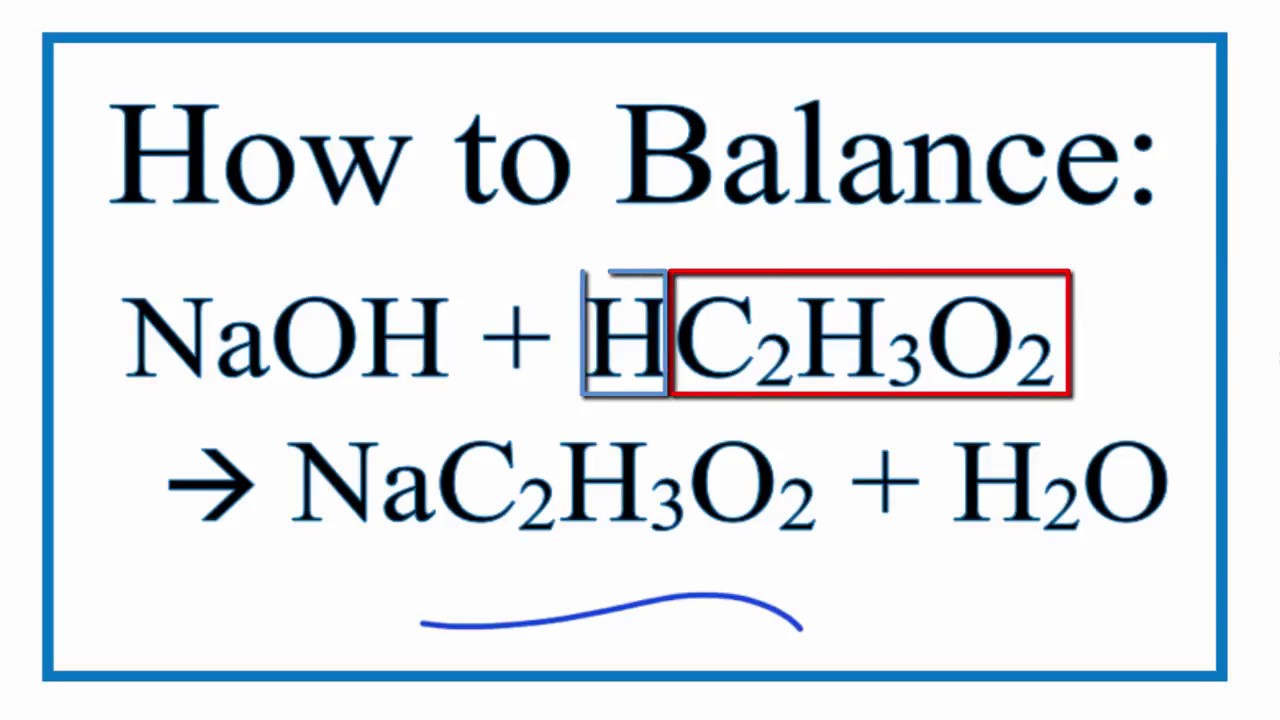
How To Balance Naoh Hc2h3o2 Nac2h3o2 H2o Sodium Hydroxide Plus Acetic Acid Youtube
2022915 0542 Mass percentage to Mass.

. 304 cm 3 319 cm 3 329 cm 3 3 317 c m 3 3. Vinegar is at least 4 acetic acid by volume making acetic acid the main component of vinegar apart from water and other trace elements. A typical resin is that produced from a polyol such as propane-123-triol glycerol with a dibasic acid such as benzene-12-dicarboxylic phthalic anhydride and a drying oil linseed or soybean oil.
On being heated together ester linkages are formed and water is a by-product. Leave the first test-tube as a control. A heterogeneous catalyst is used frequently in industry where gases flow through a solid catalyst which is often in the form of small pellets to increase the surface areaIt is often described as a fixed bed of catalyst Figure 5.
Acetone acetonitrile ACN benzene ethanol diethyl ether isopropanol methanol methyl ethyl ketone tetrahydrofuran THF. ZnO H 2 SO 4 ZnSO 4 H 2 O. One way of getting this for example would be to mix together 10 cm 3 of 10 mol dm-3 sodium ethanoate solution with 20 cm 3 of 10 mol dm-3 ethanoic acid.
C Sodium chloride from sodium sulphite and dilute hydrochloric acid. Sodium hydroxide NaOH 10 1110. The acid acts as a catalyst for the reaction between the amide and water.
2022915 0552 Mass percentage to Volume-volume percentage. Iv Write balanced equation for the following conversions. 3 a Lead sulphate from lead nitrate and sulphuric acid.
Some 3d metal compounds such as chromium hydroxide chromiumIII oxide ferric oxide has amphoteric characteristics. Amphoteric properties of chromium hydroxide CrOH 3 Chromium hydroxide CrOH 3 is an amphoteric compound and a green precipitateWhen NaOH aq is added that precipitate. Vinegar Ethanoic acid commonly called acetic acid 3.
Mass-volume percentage to Molarity Recent user inquiry. Acid base titration calculations help you identify properties such as pH of a solution during an experiment or what an unknown solution is when doing fieldwork. Amphoteric characteristics of chromium compounds.
In the following example zinc oxide becomes the zincate ion ZnOH 4 as part of soluble sodium zincate when added to concentrated base. Bases that you may know about include sodium hydroxide commonly known as caustic soda ammonium hydroxide and ammonia. Add 3 pellets of solid sodium hydroxide to the third test-tube.
Sour milk Lactic acid. Add drops of HCl to the second test-tube until the pink colour disappears. Tamarind T artaric acid 4.
Some of these are found in household cleaning products. Decorative gloss paints typically contain alkyd polymers resins. For example sodium hydroxide NaOH aq in its aqueous solutions dissociates as.
Acetic acid ə ˈ s iː t ɪ k systematically named ethanoic acid ˌ ɛ θ ə ˈ n oʊ ɪ k is an acidic colourless liquid and organic compound with the chemical formula CH 3 COOH also written as CH 3 CO 2 H C 2 H 4 O 2 or HC 2 H 3 O 2. Concentration volume 010 M 25 1000 d m 3 00025 mol Taking significant figures into account this amounts to 25 10 -3 moles. Tubular reactors are used for example in the steam cracking of ethane propane and butane and naphtha to produce alkenes.
Ethanoic acid CH3COOH 36 1045 hydrochloric acid HCl. C Concentrated sulphuric acid is added to carbon. To react with a base the amphoteric hydroxide often needs to have been freshly produced and the base must be hot and concentrated.
Bases are usually found to have a bitter taste and feel slippery soap is a good example. Average volume of diluted ethanoic acid used to neutralize the diluted sodium hydroxide sample. Divide this solution into 3 test-tubes.
The alkaline hydrolysis of amides actually involves reaction with hydroxide ions but the result is similar enough that it is still classed as hydrolysis. That is exactly what happens when amides are hydrolysed in the presence of dilute acids such as dilute hydrochloric acid. Calculating the number of moles of 01 M sodium hydroxide.
In other words the concentration of the ethanoate has to be half that of the ethanoic acid. Acids Bases and Salts 158 SCIENCE AND TECHNOLOGY Notes MODULE -. By using a solution with a known molarity and a colour indicator we measure how much of the solution is required to neutralise the unknown solution indicated by a change in the indicator which we can use to.
B Fixed bed reactors. B Nitrogen tri chloride from ammonia.

How To Write The Net Ionic Equation For Acetic Acid Sodium Hydroxide Youtube
What Is The Balanced Chemical Equation For The Neutralization Of Ch3cooh Acetic Acid And Naoh Sodium Hydroxide Quora

Net Ionic Equation For Naoh Ch3cooh Strong Base And Weak Acid

No comments for "Ethanoic Acid and Sodium Hydroxide"
Post a Comment